Assessing the Financial Impact of Translational Pharmaceutics® - Tufts CSDD Report
Unlock the benefits of the Translational Pharmaceutics® platform for your drug program
Pharmaceutical R&D activity continues to grow significantly year-on-year with increasing numbers of molecules in development. Yet despite increases in spending the industry struggles with poor R&D productivity, citing lengthy drug development times, increasing costs and high rates of molecule attrition.
The Tufts Center for the Study of Drug Development (CSDD) examined an innovative approach to accelerating drug development, Translational Pharmaceutics®, and quantified the savings to drug developers from applying the approach across the industry portfolio of investigational drugs.
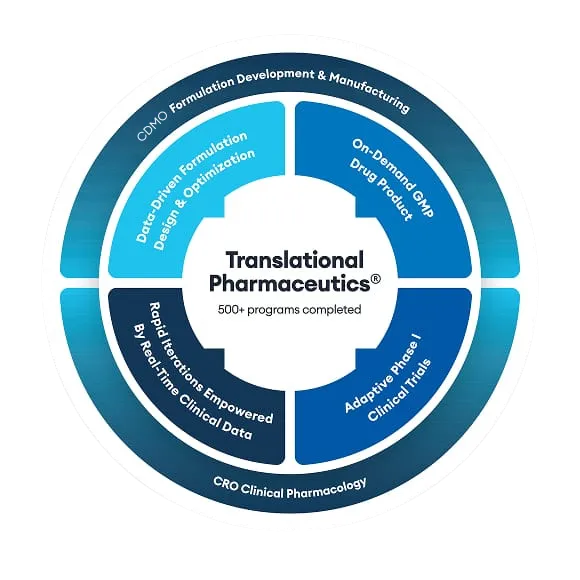
Translational Pharmaceutics® integrates real-time manufacturing and clinical testing to make drug products available for clinical trials more quickly and flexibly than is the case for traditional drug development. Translational Pharmaceutics® projects were compared to industry benchmarks, and the financial benefits were quantified on reduced industry R&D costs and increased returns from earlier sales.
In a report from the Tufts Center for the Study of Drug Development (CSDD), data were obtained for different types of Translational Pharmaceutics® projects. Topline results included mean total benefits ranging from $102.6 million to $290.1 million and mean timeline savings of >12 months per approved new drug, compared to traditional multi-vendor development paradigms.
Download a copy of the Tufts Center for the Study of Drug Development (CSDD) white paper sharing study results.