Case Study: Stealth BioTherapeutics
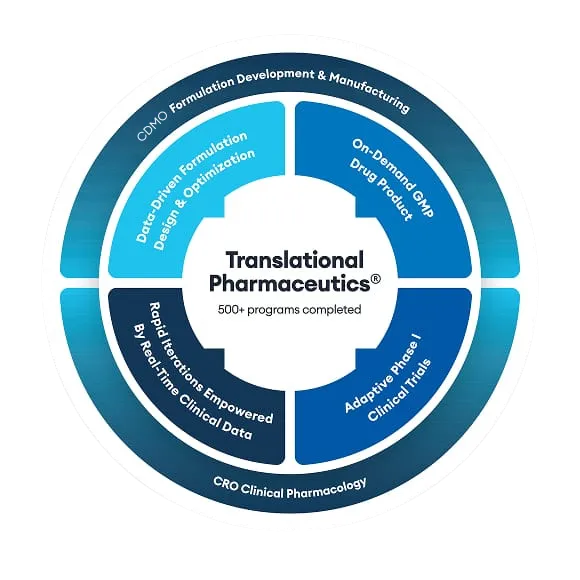
Our Translational Pharmaceutics® platform was the cost-effective, rapid delivery solution that Stealth BioTherapeutics needed to accelerate its drug development program
Stealth BioTherapeutics is an innovative biopharmaceutical company based in Massachusetts, USA, focused on developing targeted therapies for diseases linked by a common aetiology of mitochondrial dysfunction. There are more than 270 inherited orphan mitochondrial diseases that are characterized by known genetic defects. Relatively recently, research has shown that mitochondrial dysfunction is associated with conditions including heart failure, kidney disease, age-related macular degeneration, cardiovascular and metabolic diseases, neurodegeneration, and some musculoskeletal disorders.
Stealth BioTherapeutics was looking for a partner to reformulate a drug target, initially in the US, but found that option to be cumbersome in its approach to clinical manufacturing and timely potentially adding months onto their timeline. Hearing about Quotient Sciences which can offer on-site clinical manufacturing and a project design with a turnaround of 72 hours for pharmacokinetic (PK) and safety data, a site visit followed and within three days, Stealth BioTherapeutics accepted our proposal. After taking everything into account the manufacturing costs of a traditional CMO who creates multiple lots of clinical trial material that may never get used, our Translational Pharmaceutics® platform was the cost-effective option they needed.