Case Study: Druggability Technologies (DRGT)
Druggability Technologies (DRGT) needed a partner to rapidly develop a clinical formulation and commercial product
Druggability Technologies (DRGT) was a pharmaceutical company developing an array of drugs in diverse indications to achieve measurable and meaningful improvement in their clinical utility. The company used its proprietary platform to screen and select Super-API compositions and its portfolio contains over 30 preclinical and clinical-stage compounds including DRGT-46, as a novel therapy for pain.
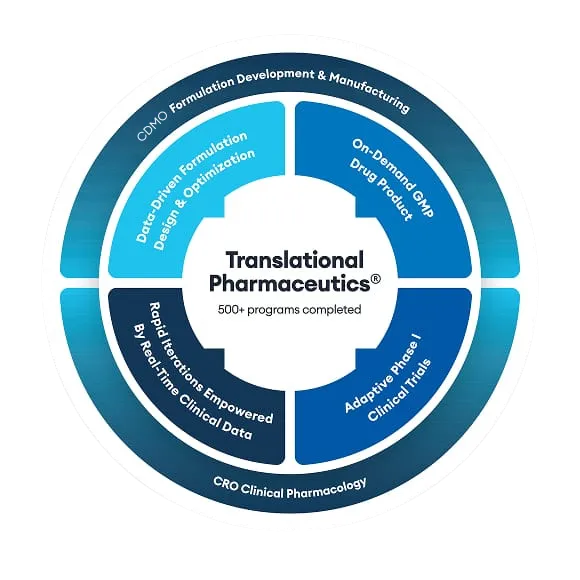
DRGT wanted to rapidly develop a clinical formulation and, if successful, a commercial product. Gábor Heltovics, former CEO of DRGT contacted Quotient Sciences to enable the rapid development, clinical assessment, and commercial readiness of their DRGT-46 product using our integrated services and network of harmonized development and manufacturing sites in the UK and the US. DRGT benefited from using our unique Translational Pharmaceutics® platform, integrating formulation development, real-time clinical manufacturing, and clinical testing to accelerate progress to scale-up. Request a copy of the case study here.