Case Study: Endevica Bio
A case study with Endevica Bio on how Translational Pharmaceutics successfully supported a novel synthetic peptide product with an integrated pharmacy compounding approach
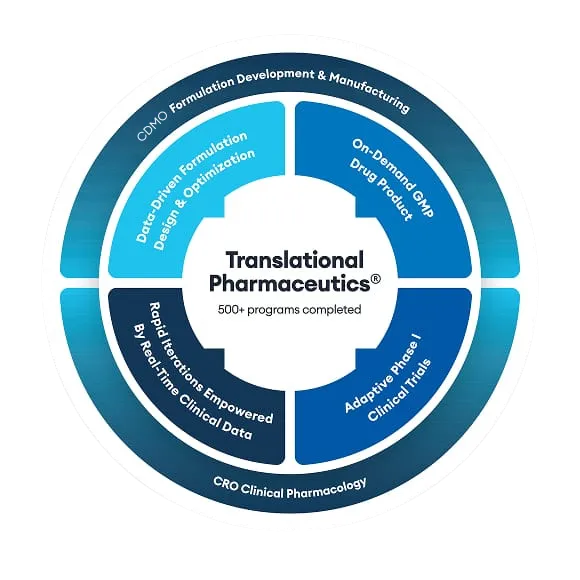
Endevica Bio, based in Chicago, USA, is a pharmaceutical company that is developing TCMCB07, a novel synthetic peptide product for the potential treatment of cachexia. This has the potential to create a new standard of care for millions of patients who develop cachexia every year.
This molecule was an ideal candidate for Quotient Sciences’ integrated Translational Pharmaceutics® platform. In our case study, Russell Potterfield, CEO of Endevica Bio, explains how Quotient Sciences has supported their business with an integrated formulation development and Phase I clinical study program, accelerating the development of TCMCB07, and saving costs in the process.
Request a copy of our case study today and contact us to find out how we can accelerate drug development timelines saving as much as 12 months in the process with our Translational Pharmaceutics® platform.