Case Study: MEI Pharma
A formulation for success. Read MEI Pharma's novel cancer therapy development case study.
MEI Pharma, headquartered in San Diego, is a clinical-stage pharmaceutical company. They are committed to the development of best-in-class cancer therapies, intended to improve outcomes for patients.
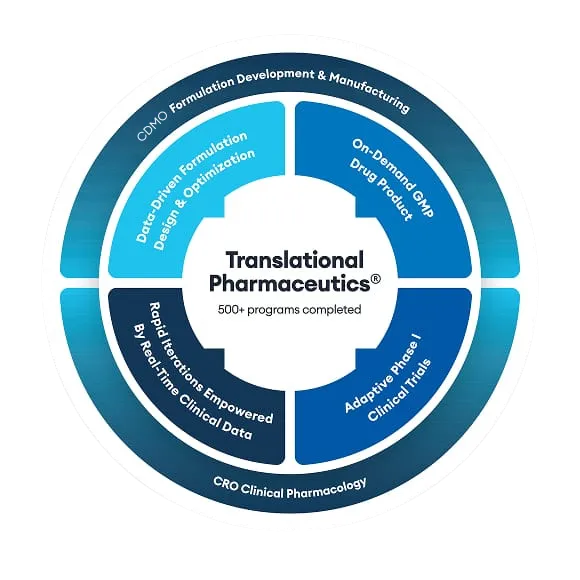
MEI Pharma leveraged Quotient Sciences’ Translational Pharmaceutics® platform to expedite the development of its next-generation cancer therapy ME-401. This is an oral PI3K delta inhibitor currently undergoing clinical trials for the treatment of recurrent chronic lymphocytic leukemia (CLL) and follicular non-Hodgkin’s lymphoma (fNHL).
Quotient Sciences' scientific experts have worked on over 400 oncology drug development projects. We offer the significant benefit of evaluating targeted oncology molecules in healthy volunteers. We can apply our Translational Pharmaceutics® platform to accelerate drug product optimization by integrating real-time drug product manufacturing and clinical assessments.
Request a copy of our case study with MEI Pharma today and find out how we can accelerate your oncology drug development timelines.