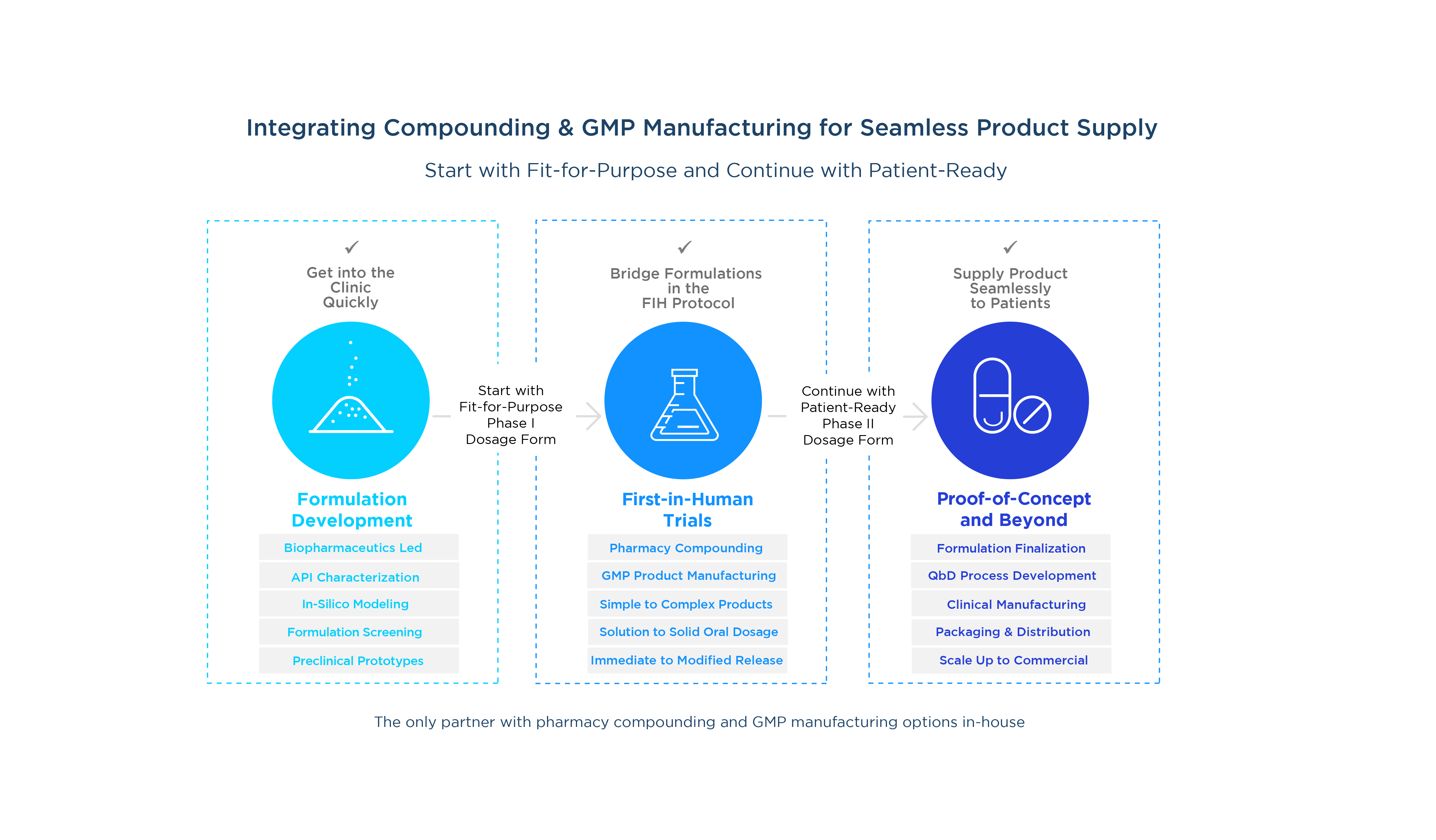
Begin your Phase I first-in-human testing with a simple, fit-for-purpose pharmacy preparation to get in the clinic quickly. See how we can help.
A fit-for-purpose pharmacy preparation
To start your first-in-human trials, we can help you develop a cost-effective dosage form that provides maximum dose flexibility to achieve your Phase I objectives.
Bridge formulations within the same FIH protocol
Evaluate formulation technologies and different dosage forms so you can select a patient-ready formulation to move forward.
Drug product to start your patient trials
Get immediate clinical trial material supply, ready for packaging and shipment, so you can start your Phase II proof-of-concept (POC) trials.
Have a challenging molecule?
We've earned a reputation for our biopharmaceutics and formulation development expertise in supporting our customers through the development of even the most complex molecules.
Leveraging our expertise and our supporting GMP manufacturing capabilities for solubility enhancement and complex oral solid dosage forms, including modified-release (MR) and immediate-release (IR) capsules and tablets, we can help achieve your goals of getting to FIH trials, then to POC.
Our solubility-enhancement technologies to produce solubilized intermediates and final dosage forms include:
- Lipidic systems
- Micronization
- Spray drying
- Hot melt extrusion

Rapidly go from FIH to POC trials
Leverage our integrated services to simplify your outsourcing and advance a patient-friendly GMP drug product into POC trials as efficiently as possible. Our compounding pharmacy is fully integrated with our pharmaceutical development and GMP manufacturing facilities located in the US and UK.