Summary: Gareth Whitaker and Chris Roe at Quotient Sciences explain how the company designs and conducts gamma scintigraphic studies to support drug development. This non-invasive imaging technique uses radiolabeled drug products to visualize in vivo behavior, such as gastrointestinal transit, drug release location, and deposition patterns.
Quotient Sciences support designing and conducting scintigraphic studies
This includes all aspects for the program, from formulation development and radiolabeling, to data analysis and program reporting.
In this article, we discuss gamma scintigraphy, a non-invasive imaging technique that incorporates a low level of gamma-emitting radionuclide into a drug product that allows drug developers to see the drug's movement within the body.
What is gamma scintigraphy?
Gamma scintigraphy is a non-invasive imaging technique that was first used in the medical industry before transitioning into clinical development.
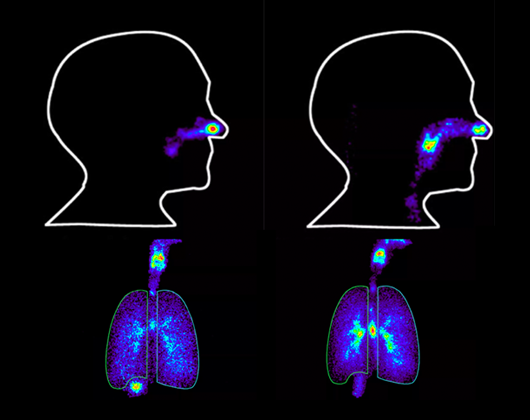
The basic principle involves incorporating a low level of gamma-emitting radionuclide (which emits energy in the form of gamma rays) into a drug product or foods and fluids, which are then administered to healthy volunteers or patients. A gamma camera is then used to detect the gamma rays being emitted, providing detailed information on the deposition, dispersion and movement of the radionuclide within the body.
The technique allows visualization and quantification of the dosed radioactivity to better understand what happens to a drug product following administration.
How do drug development teams use gamma scintigraphy?
Scintigraphic studies can be a useful tool in early development to generate data to support marketing claims for products already on the market, or close to being approved.
Gamma scintigraphy studies are valuable for proving the concept of new formulations as well as for selecting and optimizing formulations, technologies, or devices. These studies let drug developers also compare products against competitors and to evaluate pharmacodynamic responses, such as mucociliary clearance mechanisms or gastrointestinal motility.
What questions can drug developers answer by using gamma scintigraphy?
Quotient Sciences uses scintigraphy to investigate the performance of different oral solid dosage forms. Using gamma scintigraphy for oral drug products, some key questions that can be addressed are:
- How long does it take for a dosage form to transit through regions of the gastrointestinal tract?
- How long does drug release take from an oral formulation, and where has this occurred in the gastrointestinal tract?
- What effect does food and gastrointestinal pH have on an orally administered formulation?
- Does the formulation behave in vivo as predicted in vitro?
What data and images are collected using gamma scintigraphy?
Gamma cameras help development teams visualize the gamma-emitting radionuclide within clinical study participants. Following the dosing of the drug product in the clinic, regular images are taken using gamma cameras. The timing and duration of the images are determined on a case-by-case basis for each study, taking into consideration the study design, objectives, and desired outcomes.
For example, if the drug product is an immediate-release dosage form, there will be a high frequency of images taken of the subject for the first 1-2 hours following dosing. For modified release dosage forms, which are intended to release the drug over a long period of time, images could be collected over a 6-12 hour period.
What is pharmacoscintigraphy?
Scintigraphic assessments can be performed with pharmacokinetic (PK) analysis to generate an enhanced understanding of product performance. This is called pharmacoscintigraphy. Pharmacoscintigraphy can be useful in instances where interpretation of the PK data alone can present challenges, or when it is anticipated that the transit and disintegration of an oral formulation may significantly affect the PK data.
Locally-acting drug products can particularly benefit from pharmacoscintigraphy analysis. In these cases, the performance of the dosage form cannot always be assessed accurately by standard PK approaches alone, as the drug concentrations in systemic circulation may not be easily measurable.