Many of today's compounds present sub-optimal pharmacokinetic (PK) data (either predicted from in-vitro and pre-clinical data or measured in the clinic), such as poor exposure (leading to high doses), large variability, short half-life requiring more than once-a-day dosing, or Cmax-related adverse events (AEs).
Poor exposure and/or large variability can often be addressed and improved upon with enabled formulations to enhance solubility, such as an amorphous spray-dried dispersion (SDD) formulation or lipid formulations.
For compounds with large peak-to-trough ratios, more than once-a-day dosing, or Cmax-related AEs, a modified-release (MR) formulation could often be used to successfully alter the input rate and hence modify the shape of the profile to deliver the required PK exposure profile.
We help biotech and pharma customers in the development and optimization of drug products. Biopharmaceutics allows us to understand the solubility, dissolution, and permeability of a compound to identify an optimal formulation strategy.
Our chemists and formulation scientists review the properties of new drug candidates and “work their magic” to develop formulations that improve the exposure profile of the compound.
To embark on formulation optimization, be it solubility enhancement or MR development, it is key that we understand the biopharmaceutic properties of the compound to guide the formulation strategy and technology selection. Essentially, biopharmaceutics underpins the formulation strategy.
What is biopharmaceutics?
Biopharmaceutics is a relatively new scientific discipline that examines the interrelationship of the physicochemical properties of the drug, the dosage form in which the drug is given, and the route of administration on the rate and extent of systemic drug absorption (Applied Biopharmaceutics and Pharmacokinetics, Shargel, Wu-Pong and Yu, 5th Edition).
How does bioavailability play a role in biopharmaceutics?
As formulators, we want to deliver the right amount of drug at the right time with the correct concentration within the body to exert a therapeutic effect. We need to understand the systemic exposure of the drug, and for an orally administered formulation, that means understanding the process of absorption and then teasing apart the rate-limiting steps in the process.
Biopharmaceutics allows you to understand the solubility, dissolution, and permeability of a compound, and from this, we can then assess the potential fraction absorbed (Fabs). Now fraction absorbed and bioavailability are often confused and used interchangeably. Fraction absorbed is directly related to the solubility, dissolution, and permeability of a compound and is the amount of drug that enters the intestinal enterocyte in our gastrointestinal tract (FDA definition), whereas bioavailability (F) is the amount of drug in the systemic circulation able to have a therapeutic effect. F is directly related to the amount of drug absorbed (Fabs) and the amount surviving first-pass metabolism. Therefore, absorption is the input mechanism and clearance (metabolism) is the output mechanism.
As formulators, we are often able to directly impact the amount of drug absorbed through formulation optimization and improve exposure. However, the chances of improving the exposure profile of a drug that is highly cleared by formulation modification are limited.
How can biopharmaceutics help drug developers overcome challenges with their small molecules?
Understanding the biopharmaceutic properties of your compound can help you identify a formulation strategy that overcomes the challenges the compound faces or can assess the potential for the specific compound to meet the target product profile (TPP). The sooner challenging and unfixable compounds are identified and killed off in development, the less R&D expenditure will be incurred, allowing you to focus on compounds that have the legs to make it to market.
For example, if drug X has a low Fabs of 10% and F is 8%, then there is the option to increase Fabs through formulation optimization. However, if drug Y has a high Fabs (90%) but low F (e.g. 10%), even if we are able to increase absorption by another 10% (Fabs = 100%), it is unlikely to improve the exposure (F) greatly, as for drug Y clearance (metabolism) is limiting exposure. The only instances in which formulators can help in this scenario is to increase exposure (Fabs) through formulation just enough to potentially saturate the clearance mechanism. Alternatively, if the compound is subject to gut CYP3A4 metabolism, we could deliver to a lower region of the gastrointestinal tract where CYP3A4 expression is reduced, thus hoping to bypass the gut metabolism if that is the rate-limiting process for exposure. However, often in this situation, it is back to, discovery and the drawing board to revisit the compound chemistry.
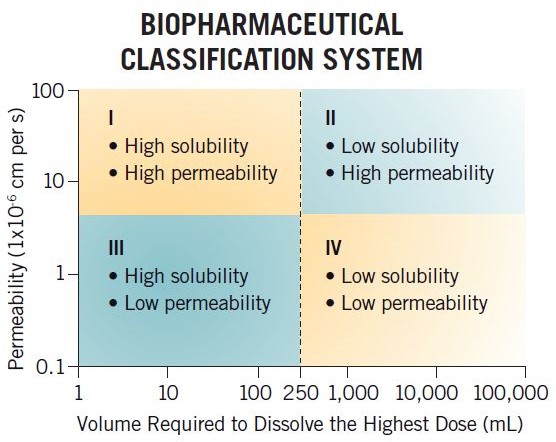
What is the Biopharmaceutics Classification System (BCS)?
The BCS is a regulatory tool that is used to justify clinical biowaivers for certain types of compounds (BCS Class I and III) based on dissolution data, allowing sponsors to justify not performing clinical bioequivalence studies when changing a formulation. The framework classifies compounds based on their permeability and solubility (buffer solubility) properties into four categories (BCS I, II, III, and IV), and this system has been used by the industry for many years to assess in-vivo performance.
For example, a BCS Class I compound with high solubility and high permeability is likely to be a good development candidate due to having high fraction absorption. However, a BCS Class IV compound is not thought of in such good light, having low permeability and low solubility and hence thought to have poor exposure. In reality, a BCS Class IV compound could have Fabs of 80% and high solubility at pH 6.5 and therefore have good Fabs and no formulation development issues.
The BCS classification criteria are strict and hence often misinform clients of their compound's formulation/development challenges. More recently, a classification system based on developability potential has been developed by Dressman and Butler, the Developability Classification System (DCS). This classifies compounds into four categories similar to the BCS but uses simulated intestinal media for the solubility assessment and also takes into consideration the compensatory nature of permeability, allowing a solubility-limited absorbable dose to be determined, which in turn allows for DCS II compounds to be divided into DCS IIa and DCS IIb compounds. DCS IIa compounds are dissolution limited and hence formulation strategies to improve exposure would focus on particle size reduction such as nanomillling and micronization, whereas DCS IIb compounds are solubility limited and hence solubility-enhancement strategies such as SDDs and lipids may be used to improve exposure.
How can the DCS be used to drive formulation strategies?
Quotient Sciences uses the DCS to help drive formulation strategies for our clients. We can either take existing customer data and assign a DCS classification or measure solubility and calculate a predicted human effective permeability (Peff) using GastroPlus® ADMET predictor, which is done by the modeling and simulation group based on the compound structure.
A recent example of this was for a compound at the candidate selection stage. Quotient Sciences supported a standalone DCS classification and formulation development package. Permeability was high and solubility at 24 hours in intestinal buffer was less than the expected therapeutic dose, so solubility was classified as “low”. However, solubility at 3 hours was found to be >10-fold higher and hence it was classified as a DCS IIa compound. So, if dissolution is rapid, absorption will be good and sophisticated solubility-enhancement strategies are not required. Quotient Sciences then developed a simple capsule formulation with particle size reduction (micronization) and wetting agents to support the first-in-human (FIH) clinical study.
In summary, biopharmaceutics underpins the formulation strategies used at Quotient Sciences, ensuring a science-based and data-driven approach to formulation optimization. This reduces the risk of drugs failing due to poor formulation and increases the chances of clinical success.
For more information about Quotient Sciences’ biopharmaceutics capabilities, contact us.

