Summary: Dr. Andrew Lewis, Chief Scientific Officer at Quotient Sciences, discusses the integration of formulation design space with Translational Pharmaceutics® to enhance oral peptide development. This approach addresses challenges like low bioavailability by optimizing formulations based on clinical data. The platform combines drug product development, manufacturing, and clinical testing, enabling just-in-time manufacturing and efficient CMC packages. This innovative strategy minimizes investment and waste, ensuring successful oral peptide programs.
Recent advancements in drug discovery, peptide engineering and drug delivery have converged to address many of the challenges associated with oral peptide delivery.
Technologies such as phage display can be used to create vast libraries of peptides that can be screened for properties of interest such as permeability and protease resistance, and numerous technologies – from permeation enhancers through to ingestible devices have been shown to be effective in promoting systemic absorption.
Drugs entering clinical trials typically start with a Phase 1, first-in-human (FIH) program that includes a single ascending dose (SAD) study - starting with a sub-therapeutic dose and gradually increasing to predicted therapeutic levels before evaluating multiple ascending doses (MAD).
As oral peptides usually have to be formulated with a drug delivery technology, multiple prototypes have to be developed with the knowledge that most of them will not be taken forward into later clinical development. Furthermore, given the many unknowns in oral peptide biopharmaceutics and poor correlation between preclinical models and humans, it is difficult to predict what the optimum formulation for performance in humans will be.
With this considered, a drug product strategy for oral peptide programs needs to be designed that enables the program objectives to be met whilst minimizing investment required and wasted API and drug product.
How does a formulation design space work?
Using the Quotient Sciences Translational Pharmaceutics® platform, we offer an innovative solution that helps mitigate these development risks—especially when applied to molecules with known challenges such as low bioavailability.
Integrating drug product development and manufacturing services with clinical testing, we enable a just-in-time manufacturing strategy for drug products that are then dosed in the clinic as they are needed. The efficient CMC packages created as part of this platform are supported by a supply chain that Quotient Sciences controls from start to finish.
With Translational Pharmaceutics®, formulations are optimized based on emerging clinical data, and using a formulation design space allows even greater flexibility. A formulation design space is a trusted concept that we have applied as part of RapidFACT® programs, an application of the Translational Pharmaceutics® platform, for almost two decades.
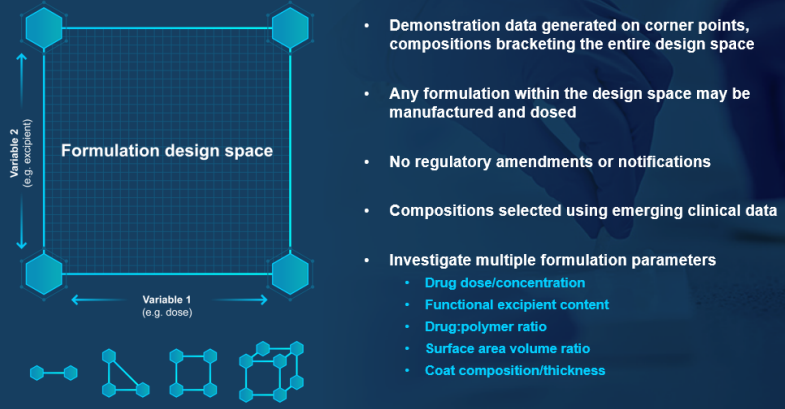
To create a formulation design space, formulation variables are identified that are anticipated to be critical-to-performance (e.g. dose and the levels of a functional excipient). Demonstration batches are manufactured at the extremes of the design space, and batch analysis and stability data are obtained for submission to the regulatory agency to gain approval to dose any formulation within that design space.
In the example shown, just four demonstration batches enable the clinical evaluation of many more formulation prototypes–without any regulatory amendments or notifications, as long as the compositions remain within the pre-defined design space.
Continue reading how we have applied the Translational Pharmaceutics® platform in the development of oral peptides, with case studies from pharma/biotech customers, in our latest whitepaper.
To get alerted about new blogs, news, and updates from our company, make sure you're on our mailing list. Subscribe for email updates.
