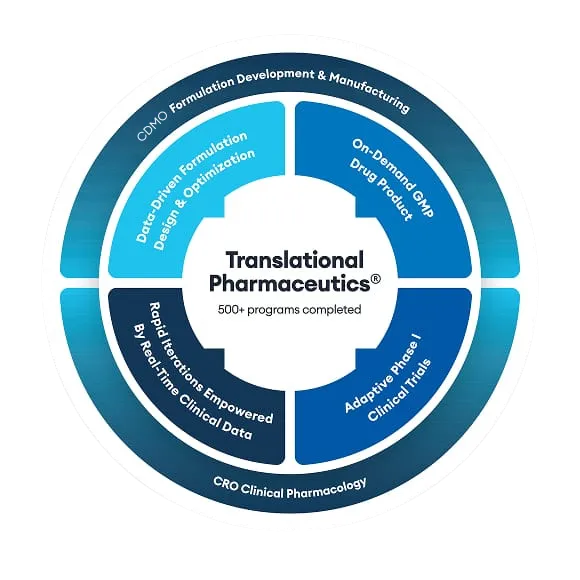
Translational Pharmaceutics® can be applied for rapid formulation and clinical testing (RapidFACT®)
Today, most new drugs require some formulation optimization during development to transition from early development formulation to one suitable for administration. Formulation optimization is also a common part of a drug lifecycle management strategy.
One application of the Translational Pharmaceutics® platform, our RapidFACT® programs, focus exclusively on drug product optimization.
Applying RapidFACT® to drug product optimization for either a new chemical entity (NCE) or during life-cycle management of an existing drug allows for the unique inclusion of a formulation design space in initial regulatory submissions and clinical protocols. This design space approach lets us iteratively optimize the composition of critical-to-performance excipients and dosage strengths relative to clinical performance to achieve your target product profile (TPP).


Over 300 programs completed.

Trusted for over 17 years.
A unique application to optimize drug products.
See how RapidFACT® programs, applied through the Quotient Sciences Translational Pharmaceutics® platform, enable a faster, integrated approach to drug development.

Maximizes flexibility
by allowing you to fine-tune drug product formulations.

Timeline acceleration
by reducing the chemistry, manufacturing, and controls (CMC) data package needed for your clinical assessments.
API Savings
that helps remove drug product scale-up from the critical path.
Explore Translational Pharmaceutics®
Beyond optimizing drug products, see how else Translational Pharmaceutics® can be applied to drug programs in these applications.
Other applications of Translational Pharmaceutics®
- Accelerating to the milestone of first-in-human clinical testing
Achieve a key milestone with our phase-specific drug formulations designed to test safety and tolerability. Utilizing Translational Pharmaceutics® for your first-in-human trials accelerates your timeline, providing critical data to support downstream development decisions.
- More efficient, integrated human ADME studies
Detect formulation issues early and get ahead of regulatory requirements for conducting human ADME studies. Our Synthesis-to-Clinic® ADME studies are enabled using Translational Pharmaceutics® for integrated drug product manufacturing and clinical testing.