
Minimizes costs
upfront related to chemistry, manufacturing, and controls (CMC).

Increases precision
in the formulations that are developed and tested, keeping you in-control and on-course of your molecule's development.

Reduces time
to get to pivotal first-in-human studies, so you can achieve the data you need and be on your way to proof-of-concept and later clinical trials more efficiently.
Explore Translational Pharmaceutics®
Beyond optimizing drug products, see how else Translational Pharmaceutics® can be applied to drug programs in these applications.
Image
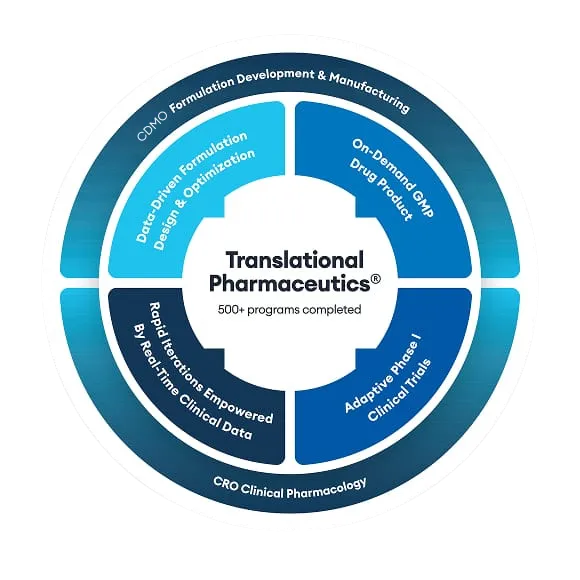
Other applications of Translational Pharmaceutics®
- Optimizing drug products following first-in-human clinical testing
We enable rapid formulation and optimization with clinical testing—simplifying reformulation after Phase I and beyond to manage product lifecycle, ensure continued innovation, and meet trial success. This is our RapidFACT® approach, an application of Translational Pharmaceutics®.
- More efficient, integrated human ADME studies
Detect formulation issues early and get ahead of regulatory requirements for conducting human ADME studies. Our Synthesis-to-Clinic® ADME studies are enabled using Translational Pharmaceutics® for integrated drug product manufacturing and clinical testing.