Dr. Paul Quigley, Principal Research Fellow, Drug Substance, discusses the ways that we can support customers with integrated strategies for scaling drug substance and drug product from early stages of molecule selection and development, through to the clinic, and beyond.
At Quotient Sciences, we engage with customers from the point of candidate drug selection, helping to ensure that the lead compounds can be manufactured in sufficient quantities to support initial toxicology and preclinical studies, while also being equipped to scale up the manufacturing processes to produce clinical drug substance to pharmaceutically acceptable, cGMP standards.
Supporting Candidate Selection to First-in-Human
When developing a drug substance manufacturing process, the chemistry of the impurities generated is as important as the purity of the compound itself. Since small differences in impurities can have a profound impact on the toxicological profile of the compound, our expertise in analytical sciences allows us to identify, quantify, and control the impurities generated in the manufacturing process. Just a few of the ways that we support customers include setting clinical phase-appropriate specifications, synthesizing analytical reference standards, and developing and validating analytical methods. Specifically, when it comes to synthetic route design, the combined experience of our scientific team ensures that the shortest, most scalable, and most economical route to drug substance can be developed.
Our synthetic chemists are constantly on the lookout for new technologies that could give us the option to shorten and simplify the drug substance development process, whether by taking stages of chemistry out of the process or tapping into commercially available raw materials that can be converted in a small number of synthetic steps to the target compound. Recent additions to our technology portfolio have included Synthetic Biology (particularly Biocatalysis), where we have several academic-sponsored studentships running. We also have a technology group in place for a three-year INNOVATE UK program with the University of Nottingham focused on continuous manufacturing using flow chemistry.
Flow technology for large-scale chemical production is not new, but recent equipment developments have enabled this to be applied on a much smaller scale. In principle, flow technology involves running reagents and solvents through a high-intensity process, usually with higher temperature and pressure in a pipe (plug flow) or continuous stirred tank (CSTR). Unlike traditional batch processes, flow technology involves the continuous feed of solvents and reagents through the reaction, minimizing the inventory of material and generally leading to higher-purity materials.
A case study (Figure 2) demonstrates the application of flow to compress several processing stages for the manufacture of 6-hydroxynorketamine (6HNK), a new non-opioid depression treatment in development by the US National Institute for Health (NIH). Hours of processing time in batch was reduced and the process itself was simplified while showing higher product purities. More importantly, we were able to process and deliver the material to the client much earlier in the clinical phase.
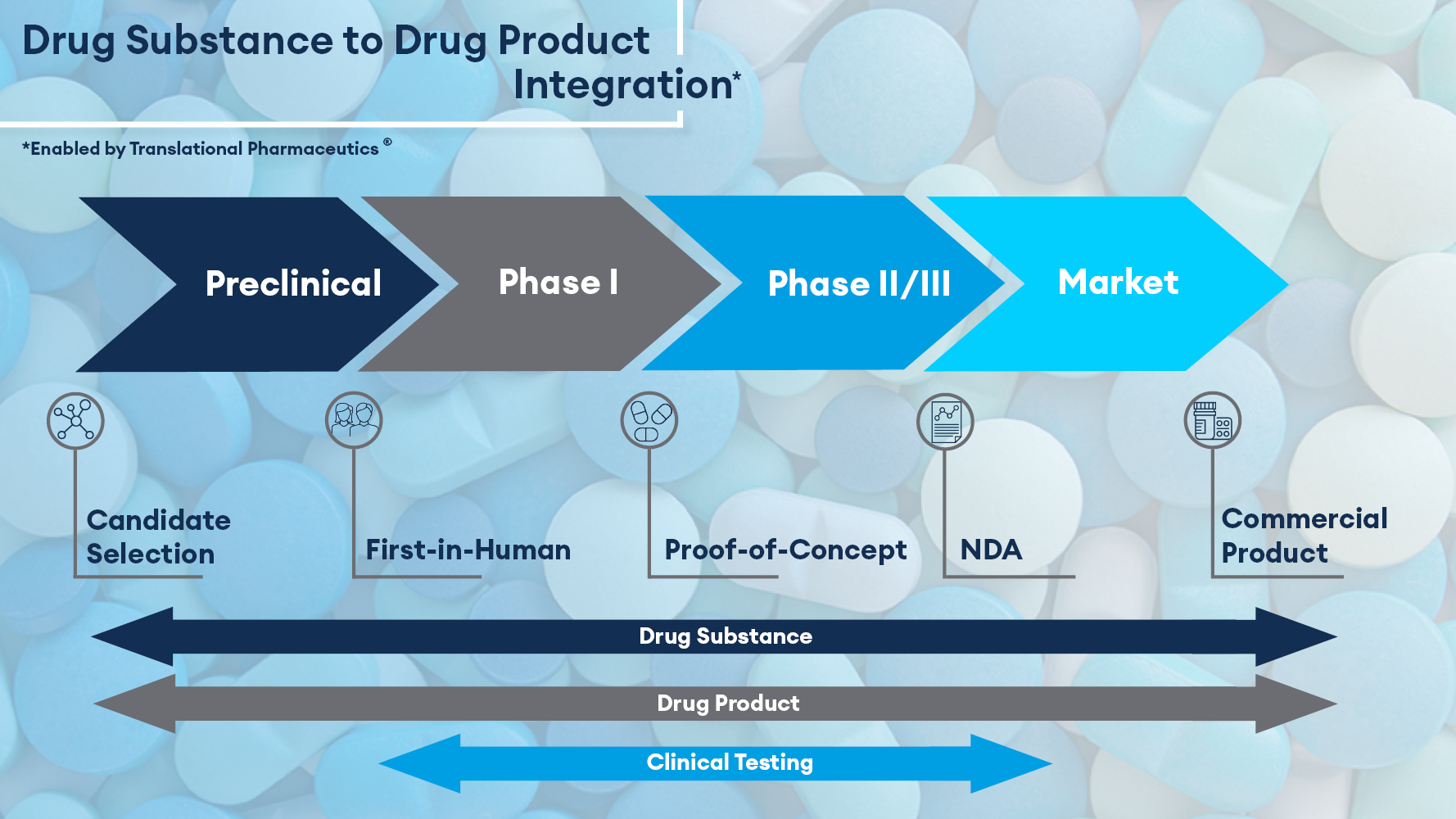
Bridging from Drug Substance to Drug Product
The drug substance-to-drug product interface is a key part of any successful drug development program. All too often, the activities relating to each are the responsibility of either different companies or siloed departments of the same organization. When multiple vendors are involved in the process, there are often questions that arise from customers including:
- Where does drug substance end?
- Where do drug product activities begin?
- Who is responsible for the grey area in between?
This creates a number of inefficiencies, and the challenges are exacerbated when we consider the complexity of today’s molecules and target product profiles, as well as the pressure to get to market faster.
Fully integrated drug substance and drug product manufacturing capabilities reduce the complexity of outsourcing while resulting in a more efficient and accelerated development plan. Timeline acceleration through ease of materials transfer and improved scheduling are among the benefits of integrating drug substance and drug product manufacturing capabilities with a single service provider.
Better workflows and processes can also be attained through improved knowledge transfer between multidisciplinary experts. For example, process chemists can work alongside analytical and solid-state chemists to ensure the rapid development and optimization of a drug substance. Likewise, formulation scientists and solid-state teams can work together to provide unambiguous data for optimizing drug substance form, which downstream leads to improved dosage form design and drug product manufacturing.
In summary, the seamless coordination between drug substance and drug product manufacturing results in a more efficient and accelerated development plan. Customers can shorten the timeline from candidate selection to clinical development an average reduction of 2-4 months or more, translating to significant R&D cost savings and accelerated time to market.
Ultimately, an integrated, agile approach to pharmaceutical development helps meet timeline pressures and gets new therapies to patients in need, faster.