Oral modified release (MR) formulations enable control over the rate and location of a drug’s release in the gastrointestinal (GI) tract, in order to achieve specific therapeutic benefits in comparison to immediate release (IR) formulations.
These benefits include maintenance of drug plasma levels over a prolonged period to reduce dosing frequency, attenuation of drug peak-to-trough ratios to lower peak-related adverse events (AEs) and improve efficacy, and drug delivery to a particular anatomical site for the treatment of local gastrointestinal (GI) disease. Therefore, drug delivery can be optimized to balance therapeutic needs, by managing AE profiles and reducing dosing frequency, both of which can contribute to improved patient compliance.
There are also commercial benefits for MR formulations that are prevalent as part of product lifecycle management (LCM). Modest reformulation of an already approved drug from an IR to MR format allows both line and patent extension opportunities and continued market exclusivity.
An impressive variety of MR formulation technologies are available, eliciting a wide range of control on drug release and delivery. Careful selection of appropriate excipients and delivery technologies are key to the design of MR formulations fulfilling specific performance requirements, from gastro-retention (GR) to sustained or pulsatile release (Table 1).
| Modified release format | Objective | Formulation technology |
| Gastro-retention |
| Swellable tablets (monolithic, bilayer, trilayer) |
| Gastric bypass |
| Enteric-coated tablets or capsules |
| Sustained or extended release |
| Matrix tablets, coated tablets, or multiparticulates |
| Targeted or controlled delivery |
| Tablets, capsules or multiparticulates |
| Biphasic release |
| Bilayer tablets or multiparticulates |
| Pulsatile release |
| Bilayer tablets or multiparticulates |
While the development of MR formulations has historically been a part of late-stage development or LCM strategies, there are increasing examples of where MR technologies have been utilized in the development of new chemical entities (NCEs). In all cases, a clear definition of the Target Product Profile (TPP) is important to outline the desired characteristics of the drug product required to deliver the desired in vivo performance. The TPP is based on the drug product requirements including the intended clinical use, dosage strength(s), drug release characteristics, stability, and other product quality criteria.
There are many MR formulation technologies that can be used to control the rate and time of drug release to achieve a particular TPP. A developer is therefore faced with the need to select the strategy that will provide optimal results in the most efficient and cost-effective manner.
Translational Pharmaceutics® for MR formulations
Selection of a specific MR platform and optimization of the quantitative levels of critical-to-performance excipients in that formulation can be challenging based on surrogate nonclinical, in vitro, or in silico data, and the recognized lack of predictability of these models to performance in humans. Traditional development also means the time and cost of taking multiple options into a clinical PK study can be prohibitive.
Translational Pharmaceutics® is a technology platform unique to Quotient Sciences, offering integrated development programs with in-study protocol flexibility to enable real-time optimization of key formulation variables based upon arising clinical data, enabling MR formulation technology platform(s) to be assessed in the identification of the best technology to achieve the desired TPP.
There are numerous potential formulation strategies available for MR dosage forms. Selecting a specific platform and the quantitative levels of critical-to-performance excipients in that formulation can be challenging based on surrogate nonclinical, in vitro, or in silico data. In-study protocol flexibility using Translational Pharmaceutics® can enable the optimization of key variables based on actual clinical data and/or the assessment of multiple technology platforms to achieve the desired TPP. Offering potential benefits in terms of PK variability and bimodal release combination flexibility, could be compared to a matrix MR tablet, which could be easier to commercialize if performance was sufficient.
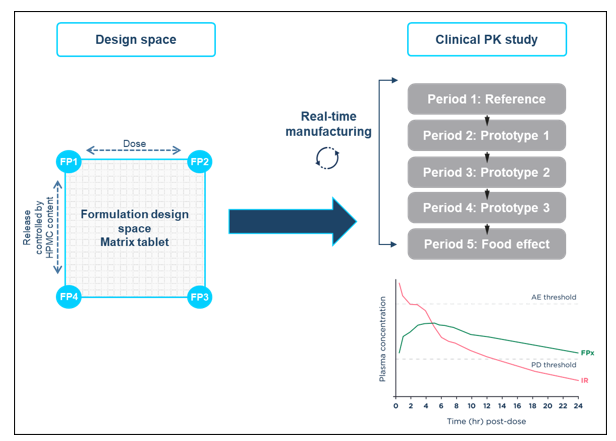
Formulation adjustments within a mapped design space included in the regulatory submission are permissible. Design space methods bracketing several formulation parameters (e.g., drug content, functional excipient content, drug:polymer ratio, surface area volume ratio, and coating composition/thickness) can be used to allow any composition within defined ranges to be selected, made, and dosed.
The design space concept can be applied to any formulation, drug product, or dosage form. The goal in MR formulations is to address all the adjustable, critical-to-performance parameters that can influence release rate and PK profile, examples of this approach can be seen in the case study delivered at Quotient Sciences which is illustrated below.
Case Study: Development of an optimized MR tablet formulation for initial proof-of-concept trials
SLx-2101, a novel PDE-5 inhibitor1 was being developed by Surface Logix as an antihypertensive agent. A Phase II pilot clinical study using an IR tablet determined it was necessary to develop a once-daily MR formulation to reduce Cmax-related AEs and ensure the 24-hour PK profile remained within the therapeutic window.
Using formulation design space concepts, a strategy built upon ICH Q8 Development Pharmaceutics, and Quality-by-Design principles, HPMC-based matrix MR tablets were developed for assessment in an adaptive relative bioavailability Phase I study to optimize the MR tablet formulation based on human clinical data. A two-dimensional formulation design space was established covering dose strengths between 10-20 mg and sustained drug release durations between approximately 12 and 20 hours.
The relationship between key formulation variables and formulation performance was investigated. Representative formulations at the extremes and the mid-points of the design space were manufactured and characterized to demonstrate that the performance of the formulation can be controlled by varying the levels of drug loading and HPMC in the formulation.
SLx-2101 MR tablet formulations within the design space were manufactured in real-time and evaluated in a flexible clinical study, avoiding the restriction of only dosing pre-defined formulation compositions. The formulation selection was driven by clinical data from the previous dosing period and the optimal MR formulation was identified in 6.5 months.
Summary
Selection of a specific MR platform can be challenging, given the lack of predictive models for human outcomes. The use of formulation design spaces, integrated manufacturing, clinical testing, and flexible clinical protocols can enable the assessment of MR platforms to de-risk development, identify the best technology to achieve the desired TPP and thereby maximize the probability of success and reduce development time, getting treatments to patients faster.
References
1. DiMasi J and Wilkinson M. The Financial Benefits of Faster Development Times: Integrated Formulation Development, Real-Time Manufacturing, and Clinical Testing. TIRS, June 2020.
2. USFDA. Conference on Harmonization (ICH) and FDA Guidance for Industry, Q8 (R2) Pharmaceutical Development 2009. https://www.fda.gov/media/71535/download. Accessed May 30, 2019.
3. McDermott J, Scholes P. Formulation design space: a proven approach to maximize flexibility and outcomes within early clinical development. Therapeutic Delivery. 2015;6(11):1269-1278. doi.org/10.4155/tde.15.76.
4. Lin, W, et al. Development of a Formulation Design Space for SLx-2101 Modified Release Tablets to Enable a Flexible Phase I Pharmacokinetic Study (Controlled Release Society Annual Meeting 2010).