Perform FIH predictions
to predict fraction absorbed, maximum absorbable dose, starting dose, and potential dose range of your drug product

Conduct quality-by-design (QbD) assessments
to assess the impact of changes to your drug product’s particle size, shape, or dissolution rate

Predict outcomes in special populations
including pediatrics, renal or hepatic impairment, and diverse ethnicities, to better inform and conduct virtual clinical trials, such as bioequivalence assessments
Why choose Quotient Sciences for modeling & simulation services?
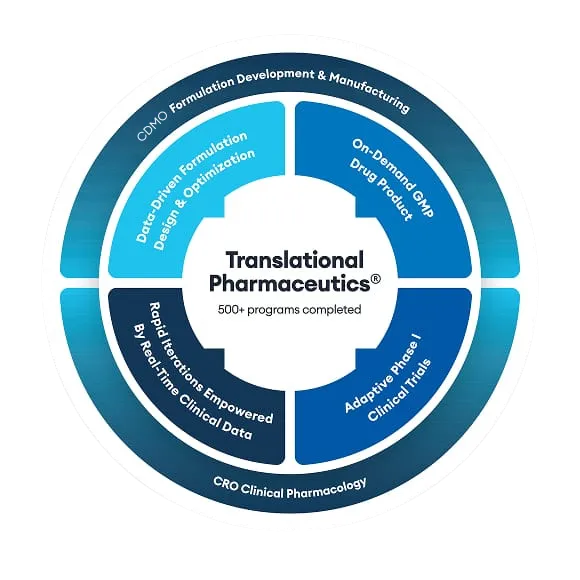
Integrating modeling & simulation activities with a Translational Pharmaceutics® program
Using Translational Pharmaceutics®, your first-in-human clinical program or drug optimization program benefits from the simulation of exposure profiles, which help to select doses and define robust formulation strategies.
Using GastroPlus™ and other tools, we can model your drug product’s physicochemical, biopharmaceutic, and drug metabolism and pharmacokinetic data.
Learn more about how we can apply Translational Pharmaceutics® along with modeling & simulation services to your next drug program.