Accelerate molecules through development by integrating traditionally siloed services.
The Quotient Sciences Translational Pharmaceutics® platform is a disruptive approach to drug development that helps you forge your own path to success and optimize every step by redefining the complementary, interconnected relationship between drug product design, supply and clinical testing.
Translational Pharmaceutics® integrates activities and adapts solutions to help you reach key milestones as quickly and efficiently as possible.
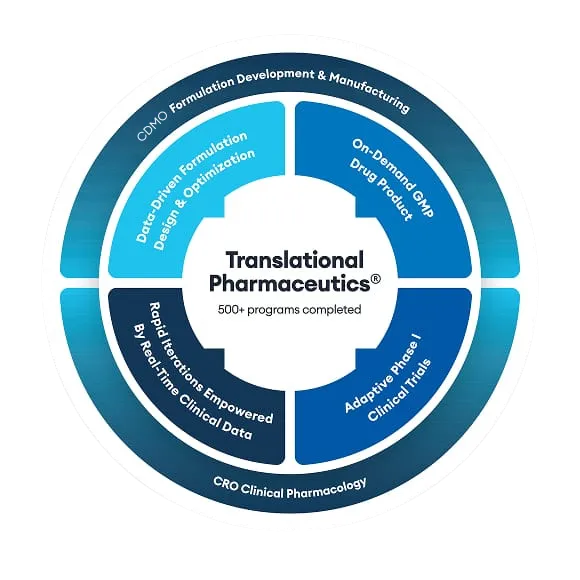

Redefining Drug Development
Over 17 years and 500+ programs, we’ve pioneered the integration of CRO and CDMO solutions. We merge operational efficiencies, scientific rigor, and clinical insights into a single program of work that is delivered under a single project manager and organization.

Enabling Acceleration
We bypass drug-development silos. Our comprehensive approach to formulation development, on-demand GMP manufacturing, and clinical testing leverages actionable data to empower successful outcomes—manufacturing and releasing drug products in less than seven days and reducing overall development timelines by 9-12 months on average.

Integrating Efficiency
Close integration between drug product manufacturing and clinical testing enables better decision-making while reducing time and waste. Our phase-appropriate expertise anticipates and overcomes challenges to create a personalized path for your molecule.
Benefits of integrated drug development with Translational Pharmaceutics®
See how Quotient Sciences enables a faster, integrated approach to drug development.
Streamlines & simplifies
vendor management & supply chain

Provides flexibility
to adjust formulation composition within a study

Better decisions
based on emerging human clinical data
Cost savings
in R&D spend

Timeline acceleration
by 12 months or more

Conserves API
for high-value drug substance
In control, on course and always one step ahead—no matter your development phase.
Translational Pharmaceutics® helps you develop your drug—from first in human (FIH) studies to managing your ongoing product lifecycle. Learn more about the unique applications of the Quotient Sciences Translational Pharmaceutics® platform.
- Translational Pharmaceutics® for accelerating first-in-human, Phase I clinical testing
Reach a pivotal milestone with our phase-specific drug product to test the safety and tolerability of your drug. When Translational Pharmaceutics® is applied for your first-in-human clinical trials, you can accelerate to the clinic and see critical data faster to help inform development decisions downstream.
- Translational Pharmaceutics® for drug product optimization in Phase Ib/IIa clinical studies
We enable rapid formulation and optimization with clinical testing—simplifying reformulation after Phase I and beyond to manage product lifecycle, ensure continued innovation, and meet trial success. This is our RapidFACT® approach, an application of Translational Pharmaceutics®.
- Translational Pharmaceutics® for more efficient, integrated human ADME studies
Whether conducting human ADME for New Drug Applications (NDA) or running in parallel with Phase II Proof of Concept (POC) studies, our Synthesis-to-Clinic® integrated ADME studies are enabled by applying Translational Pharmaceutics®.
By the numbers

Trusted for over 17 years
as a proven method to integrate drug development and streamline process steps.

100+ customers
globally have used the Translational Pharmaceutics® platform.

More than 500 programs
completed in therapy areas including oncology, neurology (CNS), and pediatrics.
What our customers say about Translational Pharmaceutics®
Meet Quotient Sciences Translational Pharmaceutics® Experts
With decades of industry and drug development knowledge, meet some of our team members who can help you navigate questions about the integration of drug product manufacturing with clinical testing and realize the benefits of the Translational Pharmaceutics® platform for your next drug program.
Dr. Andrew Lewis
Chief Scientific Officer
Dr. Andrew (Andy) Lewis is the Chief Scientific Officer at Quotient Sciences. As the leader of Quotient Sciences' scientific teams...
About AndrewJohn McDermott
VP, Scientific Consulting
John McDermott leads Quotient Sciences' global drug development consulting, research fellows, modeling & simulation, and clien...
About JohnDr. Vanessa Zann
VP, Scientific Consulting, Translational Pharmaceutics & Clinical Pharmacology - USA
Dr. Vanessa Zann has over twenty-five years industry experience providing expert biopharmaceutic support to drug discovery, early ...
About VanessaDr. Andrew Parker
Senior Drug Development Consultant
Dr. Andrew Parker has over two decades of experience in the pharmaceutical industry, spanning from preclinical development, throug...
About Andrew