Partner with us to design and deliver your clinical pharmacology program.
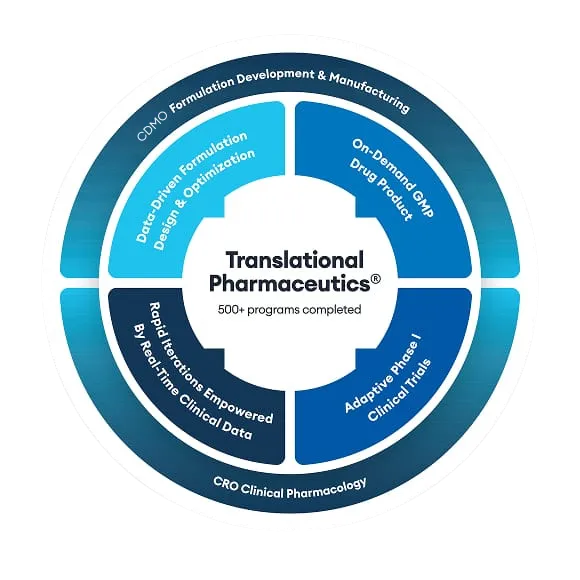
Quotient Sciences is dedicated to Phase I trials and early drug development, helping you accelerate your molecule from first-in-human (FIH) trials to proof of concept (POC). We focus on providing the insights you need by offering comprehensive clinical pharmacology services for all Phase I study programs.
Our services encompass expert clinical study design, thorough data analysis, and detailed reporting. With our fully integrated approach, we ensure seamless execution and deliver high-quality results safely and quickly.