What are some of the key considerations in the selection of a candidate compound?
Before setting out on a journey traveling to a destination, it is normal to do some research. You may rely on previous knowledge, look at a map, consider the type of transport, and find out what the likely disruptions or hazards are on your route to ensure they can be avoided.
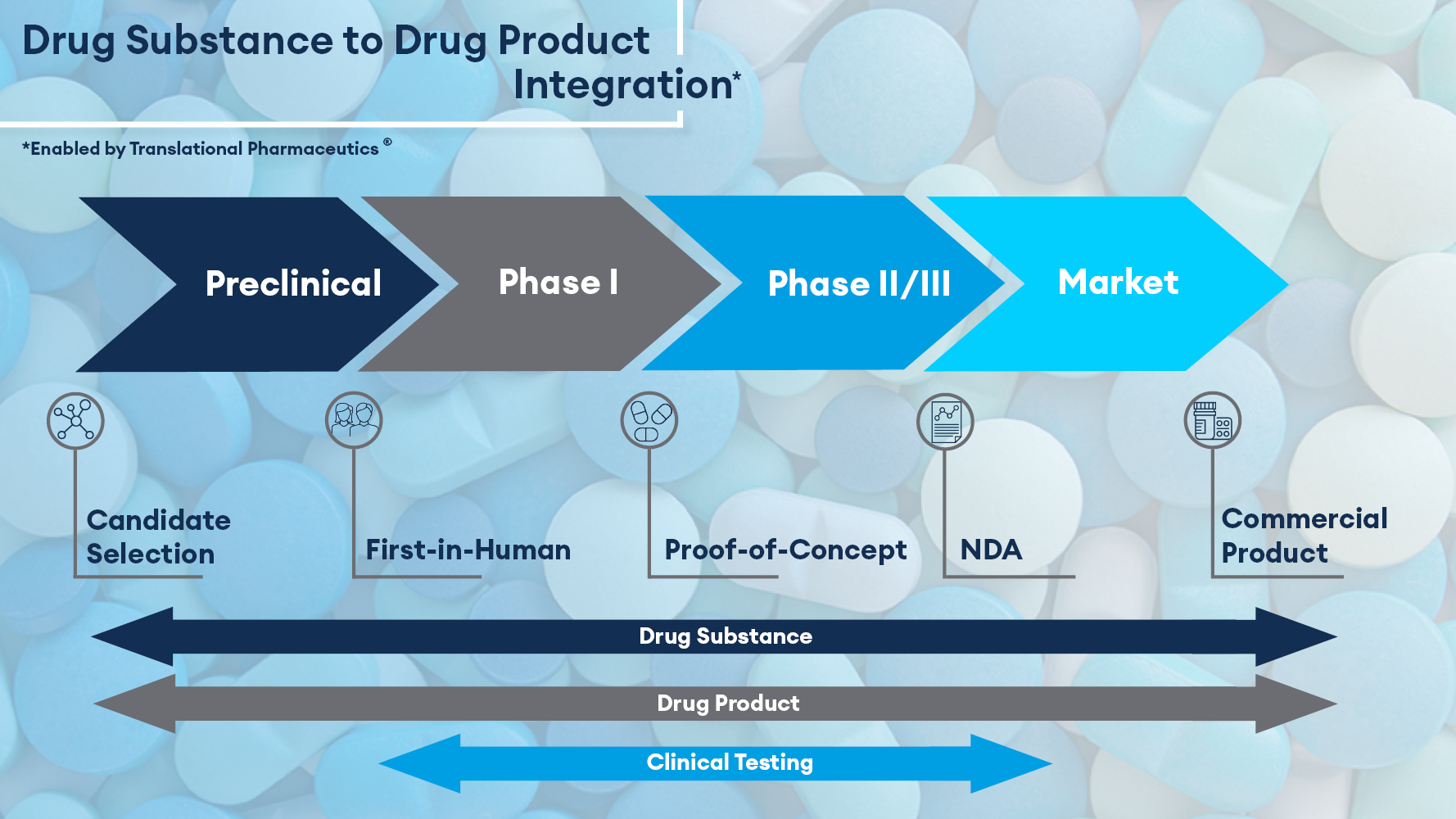
Developing a drug is also a journey. You will need to develop an understanding of your candidate compound and have an idea of your destination; what is the target disease, the patient group, the likely route of administration, the target product profile, and importantly, what are the potential pitfalls along the way?
From the outset, if for example, your compound is inherently unstable, has very poor solubility, or is hygroscopic, you know your product development journey is going to be more challenging. Therefore, finding people with the right knowledge, skills, and technologies will be important to identify and overcome potential obstacles. The earlier in your journey that these problems are identified the better. The simplest and most effective pathways can be established, reducing both the time and cost of your development, or when appropriate the development of a compound can be stopped and efforts focused on other molecules which show more promise.
After a series of lead compounds are identified, a selection process takes place to prioritize which compound(s) to take forward. Pharmaceutical and biotechnology companies take different approaches to their early development work, and the approach chosen may be based on their internal experience, resources, culture, budget, timeline, availability of API, and their attitude to risk.
In our 30 years of drug development experience, we observed that delaying the basic characterization of a compound may lead to significant issues later in development.
Our approach to drug product development
Quotient Sciences adopts a holistic approach with multidisciplinary development teams of experts in biopharmaceutics, DMPK, materials science, and formulation development, brought together to help guide the process. In early-stage screening, our flexible approaches are always tailored to the molecule and the delivery route, data-driven and technology-agnostic. We are mindful that strategies need to be fit-for-phase, cost-effective, and rapid.
For solid oral delivery systems, it is important to first characterize the solid-state properties of the input API using a range of analytical techniques. We recognize that at the very early stages of development, API can be in short supply, and the synthesis of multiple batches may yield slightly different properties, including the degree of crystallinity, polymorphism, purity, particle size, morphology, and wettability. These variations may affect your initial toxicity studies and therefore understanding your early batches will assist with troubleshooting and specification setting once a validated synthetic route is established.
Often at this stage, the API is amorphous or only partially crystalline. Therefore, one of the main early drivers of a project is to obtain a crystalline form to allow purification of the API as part of the API manufacturing process and so we are often tasked with performing an initial crystallization screen as a first step. These quick screens are used to obtain an early indication of the polymorphic tendency of the molecule.
Next, a solubility screen would likely be initiated looking at solubility in differing pH, biorelevant media, and common process solvents. If solubility is low and likely to affect bioavailability, it would be prudent to examine the possibility of solubility enhancement via modification of the solid-state properties or salt form of the API, before adopting additional formulation enhancement solutions to improve solubility (for example amorphous solid dispersions).
If the API has ionizable groups, a salt screen may also be considered. Modifying a free form or changing salt form can impart many advantageous properties of API, not least solubility, crystallinity, and stability. We design our salt screens with your molecule at the core, the counter ions to be studied are chosen based on several factors, not just the pKa of your molecule. We also consider molecular weight, likely dose strength, and the possible formulation process, to ensure optimal outcome from a wide variety of conditions using only minimal API quantities.
This screening approach is favored at Quotient Sciences because of its rapid turnaround time and typically it only uses milligram API quantities per experiment, whilst still enabling a meaningful characterization to be performed. Based on the results obtained, potential hits are then prioritized for scale-up to allow more detailed solid-state characterization, selection, and comparison to the initial input API.
At some stage in the development process, an understanding of any potential polymorphism of your API is likely to be required, as this can affect many important downstream considerations including the quality, manufacturability, bioavailability, safety, and performance of your product. The timing of polymorphism characterization may depend on API supply, timelines, and budget, however, in our experience, this should be performed as early as possible. Unexpected changes in the API during formulation development or manufacture can result in costly and time-consuming delays to development plans.
Like solid-state characterization, our polymorphism screens are designed with the API in mind. While initially there may appear to be a limitless possibility of solvents to use, our experience has demonstrated that by using routine process solvents/solvent mixture systems and concentrating on multiple kinetic and thermodynamic crystallization processes, the most relevant forms will be identified. The other advantage to this approach, compared to other high throughput solvent-mediated screens, is that it allows for the identification of formulation processing issues so that these may be mitigated against before the costly formulation development takes place.
Once a candidate has been selected, additional testing can also be employed. For example, accelerated forced degradation studies can be performed to ensure the integrity of the chosen form, and initial excipient compatibility screens can be performed to identify possible adverse interactions during formulation development. Our Modelling and Simulation team can also perform physiologically based pharmacokinetic (PBPK) studies to provide a mechanistic understanding of the likely performance and disposition of your drug product in vivo and inform risk-based decisions on both the formulation and clinical design, ultimately reducing the cost of development.
What are the typical solid-state characterization approaches used in the screens?
- Polarized light microscopy - allows us to rapidly determine and easily see if a crystalline material is present. Additional information about the crystal's habit and size also gives vital information to help inform decisions and planning.
- X-ray powder diffraction - is used to determine the crystal form and the signature patterns can be used to track the solid form through different formulation processes and throughout stability.
- Dynamic vapor sorption - gives vital information on the hygroscopicity of the compound and hydrate formation. We also routinely use dynamic vapor sorption to understand the wettability of the API and batch-to-batch variability due to subtle changes in crystal habit. These properties are key in dictating future storage and processing conditions.
- Thermogravimetric analysis - is used to measure weight changes over a temperature range. This is useful for determining the presence of hydrates or solvates and to determine degradation temperatures when using heat-induced processing conditions.
- Differential Scanning Calorimetry - is used to determine the melting point and purity of our material. It can also be used for our initial excipient compatibility screening studies and glass transition determination for amorphous solid dispersions screens/characterization.
- Confocal Raman Spectroscopy and mapping - are used to identify the solid form within formulations, and investigate changes during stability and are generally an excellent tool to aid investigations into formulation, stability, or processing problems.
- Solution and perfusion Calorimetry - are the techniques to measure low-level discriminatory surface disorder and amorphous content, again particularly needed for inhalation compounds to ensure amorphous content post micronization is controlled and understood.
Why is this relevant for the future development of the compound?
Understanding and controlling the solid-state properties of your API is a key part of the candidate selection and development journey.
Early in development, modest investment in these initial studies and guidance from experienced scientists will help you on your drug development journey. You will be able to build up knowledge of your API, identify potential pitfalls, and ultimately de-risk your development program, getting you to the final product destination as quickly and efficiently as possible.